Software Validation Procedure Iso 13485 Template. How Ideagen can help with the validation. No software validation procedure on this planet will save you when youve hired the wrong people.
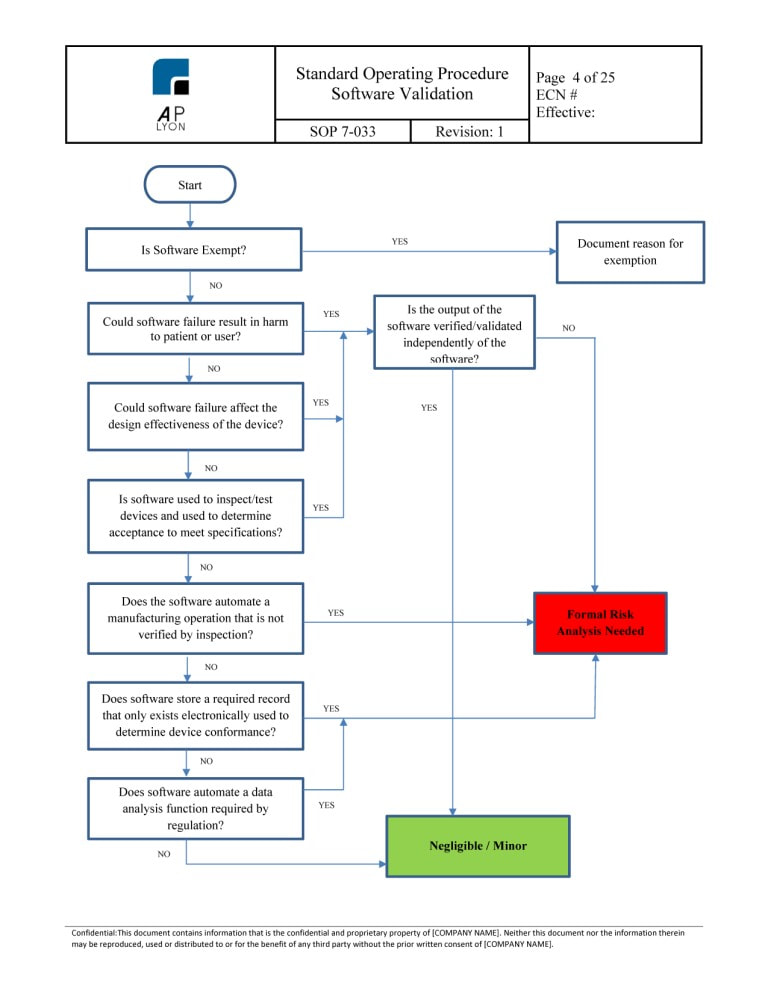
Ensure that the. The new requirement in section 416 reads. The Medical Device Software Procedure Bundle includes procedures related to development of software products validation of software software clinical evaluation and how to apply human factors and usability engineering to the medical device development process.
Also includes Medical Device Software Development Plan.
The Validation Master Plan VMP comes with other documents. The Medical Device Software Procedure Bundle includes procedures related to development of software products validation of software software clinical evaluation and how to apply human factors and usability engineering to the medical device development process. Validation of Software used for Monitoring and Measurement. Requirements of ISO 13485.